Химическое равновесие в гомогенных системах (решение задач)
52. (4/1-97).Для процесса диссоциации идеального газа А2 = 2А выразить в явном виде зависимость константы равновесия KP от степени диссоциации a
, измеряемой в изобарном и изохорном процессах. При каком начальном давлении Po(A2) будет достигаться a
= 0,5 в этих случаях, если KP = 1 бар?
Решение.
При проведении процесса в изобарных условиях:
KP =
P
A
2
P
A
2
=
P
Σ
(2ξ)
2
(
n
0
−ξ)(
n
0
+ξ)
=
P
Σ
(2ξ)
2
(
n
0
)
2
−
ξ
2
=
P
Σ
(2α)
2
1−
α
2
MathType@MTEF@5@5@+=feaagaart1ev2aaatCvAUfeBSjuyZL2yd9gzLbvyNv2CaerbuLwBLnhiov2DGi1BTfMBaeXatLxBI9gBaerbd9wDYLwzYbItLDharqqtubsr4rNCHbGeaGqiVu0Je9sqqrpepC0xbbL8F4rqqrFfpeea0xe9Lq=Jc9vqaqpepm0xbba9pwe9Q8fs0=yqaqpepae9pg0FirpepeKkFr0xfr=xfr=xb9adbaqaaeGaciGaaiaabeqaamaabaabaaGcbaWaaSaaaeaacaWGqbWaa0baaSqaaiaadgeaaeaacaaIYaaaaaGcbaGaamiuamaaBaaaleaacaWGbbWaaSbaaWqaaiaaikdaaeqaaaWcbeaaaaGccqGH9aqpcaWGqbWaaSbaaSqaaiabfo6atbqabaGcdaWcaaqaaiaacIcacaaIYaGaeqOVdGNaaiykamaaCaaaleqabaGaaGOmaaaaaOqaaiaacIcacaWGUbWaaWbaaSqabeaacaaIWaaaaOGaeyOeI0IaeqOVdGNaaiykaiaacIcacaWGUbWaaWbaaSqabeaacaaIWaaaaOGaey4kaSIaeqOVdGNaaiykaaaacqGH9aqpcaWGqbWaaSbaaSqaaiabfo6atbqabaGcdaWcaaqaaiaacIcacaaIYaGaeqOVdGNaaiykamaaCaaaleqabaGaaGOmaaaaaOqaaiaacIcacaWGUbWaaWbaaSqabeaacaaIWaaaaOGaaiykamaaCaaaleqabaGaaGOmaaaakiabgkHiTiabe67a4naaCaaaleqabaGaaGOmaaaaaaGccqGH9aqpcaWGqbWaaSbaaSqaaiabfo6atbqabaGcdaWcaaqaaiaacIcacaaIYaGaeqySdeMaaiykamaaCaaaleqabaGaaGOmaaaaaOqaaiaaigdacqGHsislcqaHXoqydaahaaWcbeqaaiaaikdaaaaaaaaa@6C4E@
,
откуда при KP = 1 и α = 0,5, P0(A2) = PS
= 0,75 бар.
Если процесс вести изохорно, то в равновесном состоянии
KP =
P
A
2
P
A
2
=
RT
V
(2ξ)
2
(
n
0
−ξ)
MathType@MTEF@5@5@+=feaagaart1ev2aaatCvAUfeBSjuyZL2yd9gzLbvyNv2CaerbuLwBLnhiov2DGi1BTfMBaeXatLxBI9gBaerbd9wDYLwzYbItLDharqqtubsr4rNCHbGeaGqiVu0Je9sqqrpepC0xbbL8F4rqqrFfpeea0xe9Lq=Jc9vqaqpepm0xbba9pwe9Q8fs0=yqaqpepae9pg0FirpepeKkFr0xfr=xfr=xb9adbaqaaeGaciGaaiaabeqaamaabaabaaGcbaWaaSaaaeaacaWGqbWaa0baaSqaaiaadgeaaeaacaaIYaaaaaGcbaGaamiuamaaBaaaleaacaWGbbWaaSbaaWqaaiaaikdaaeqaaaWcbeaaaaGccqGH9aqpdaWcaaqaaiaadkfacaWGubaabaGaamOvaaaadaWcaaqaaiaacIcacaaIYaGaeqOVdGNaaiykamaaCaaaleqabaGaaGOmaaaaaOqaaiaacIcacaWGUbWaaWbaaSqabeaacaaIWaaaaOGaeyOeI0IaeqOVdGNaaiykaaaaaaa@49B9@
=
n
0
RT
V
(2α)
2
(1−α)
=
P
A
2
нач
(2α)
2
(1−α)
MathType@MTEF@5@5@+=feaagaart1ev2aaatCvAUfeBSjuyZL2yd9gzLbvyNv2CaerbuLwBLnhiov2DGi1BTfMBaeXatLxBI9gBaerbd9wDYLwzYbItLDharqqtubsr4rNCHbGeaGqiVu0Je9sqqrpepC0xbbL8F4rqqrFfpeea0xe9Lq=Jc9vqaqpepm0xbba9pwe9Q8fs0=yqaqpepae9pg0FirpepeKkFr0xfr=xfr=xb9adbaqaaeGaciGaaiaabeqaamaabaabaaGcbaWaaSaaaeaacaWGUbWaaWbaaSqabeaacaaIWaaaaOGaamOuaiaadsfaaeaacaWGwbaaamaalaaabaGaaiikaiaaikdacqaHXoqycaGGPaWaaWbaaSqabeaacaaIYaaaaaGcbaGaaiikaiaaigdacqGHsislcqaHXoqycaGGPaaaaiabg2da9iaadcfadaqhaaWcbaGaamyqamaaBaaameaacaaIYaaabeaaaSqaaiaad2dbcaWGWqGaam4reaaakmaalaaabaGaaiikaiaaikdacqaHXoqycaGGPaWaaWbaaSqabeaacaaIYaaaaaGcbaGaaiikaiaaigdacqGHsislcqaHXoqycaGGPaaaaaaa@5335@
.
После подстановки KP = 1 и α = 0,5 находим: P0(A2) = 0,5 бар.
67. (3/1-99)*. Для реакции диссоциации Br2 (газ) = 2Br (газ)
зависимость константы равновесия от температуры в единицах СИ имеет вид: ln KP = –23009/T + 0,663 lnT + 8,12
Оценить энергию связи в молекуле Br2.
Решение.
K
P
=exp(
−
Δ
r
G
0
RT
)=exp(
−
Δ
r
H
0
(T)
RT
)exp(
Δ
r
S
0
(T)
R
)
MathType@MTEF@5@5@+=feaagaart1ev2aaatCvAUfeBSjuyZL2yd9gzLbvyNv2CaerbuLwBLnhiov2DGi1BTfMBaeXatLxBI9gBaerbd9wDYLwzYbItLDharqqtubsr4rNCHbGeaGqiVu0Je9sqqrpepC0xbbL8F4rqqrFfpeea0xe9Lq=Jc9vqaqpepm0xbba9pwe9Q8fs0=yqaqpepae9pg0FirpepeKkFr0xfr=xfr=xb9adbaqaaeGaciGaaiaabeqaamaabaabaaGcbaGaam4samaaBaaaleaacaWGqbaabeaakiabg2da9iGacwgacaGG4bGaaiiCamaabmaabaGaeyOeI0YaaSaaaeaacqqHuoardaWgaaWcbaGaamOCaaqabaGccaWGhbWaaWbaaSqabeaacaaIWaaaaaGcbaGaamOuaiaadsfaaaaacaGLOaGaayzkaaGaeyypa0JaciyzaiaacIhacaGGWbWaaeWaaeaacqGHsisldaWcaaqaaiabfs5aenaaBaaaleaacaWGYbaabeaakiaadIeadaahaaWcbeqaaiaaicdaaaGccaGGOaGaamivaiaacMcaaeaacaWGsbGaamivaaaaaiaawIcacaGLPaaaciGGLbGaaiiEaiaacchadaqadaqaamaalaaabaGaeuiLdq0aaSbaaSqaaiaadkhaaeqaaOGaam4uamaaCaaaleqabaGaaGimaaaakiaacIcacaWGubGaaiykaaqaaiaadkfaaaaacaGLOaGaayzkaaaaaa@5EA2@
ln
K
P
=−
Δ
r
H
0
(T)
RT
+
Δ
r
S
0
(T)
R
=−
Δ
r
H
298
0
+
Δ
r
c
p
⋅(
T−298
)
RT
+
Δ
r
S
298
0
+
Δ
r
c
p
⋅ln
T
298
R
=
=−
Δ
r
H
298
0
−298⋅
Δ
r
c
p
RT
+
Δ
r
c
p
R
lnT+(
Δ
r
c
p
(
1−ln298
)+
Δ
r
S
298
0
R
).
MathType@MTEF@5@5@+=feaagaart1ev2aaatCvAUfeBSjuyZL2yd9gzLbvyNv2CaerbuLwBLnhiov2DGi1BTfMBaeXatLxBI9gBaerbd9wDYLwzYbItLDharqqtubsr4rNCHbGeaGqiVu0Je9sqqrpepC0xbbL8F4rqqrFfpeea0xe9Lq=Jc9vqaqpepm0xbba9pwe9Q8fs0=yqaqpepae9pg0FirpepeKkFr0xfr=xfr=xb9adbaqaaeGaciGaaiaabeqaamaabaabaaGceaqabeaaciGGSbGaaiOBaiaadUeadaWgaaWcbaGaamiuaaqabaGccqGH9aqpcqGHsisldaWcaaqaaiabfs5aenaaBaaaleaacaWGYbaabeaakiaadIeadaahaaWcbeqaaiaaicdaaaGccaGGOaGaamivaiaacMcaaeaacaWGsbGaamivaaaacqGHRaWkdaWcaaqaaiabfs5aenaaBaaaleaacaWGYbaabeaakiaadofadaahaaWcbeqaaiaaicdaaaGccaGGOaGaamivaiaacMcaaeaacaWGsbaaaiabg2da9iabgkHiTmaalaaabaGaeuiLdq0aaSbaaSqaaiaadkhaaeqaaOGaamisamaaDaaaleaacaaIYaGaaGyoaiaaiIdaaeaacaaIWaaaaOGaey4kaSIaeuiLdq0aaSbaaSqaaiaadkhaaeqaaOGaam4yamaaBaaaleaacaWGWbaabeaakiabgwSixpaabmaabaGaamivaiabgkHiTiaaikdacaaI5aGaaGioaaGaayjkaiaawMcaaaqaaiaadkfacaWGubaaaiabgUcaRmaalaaabaGaeuiLdq0aaSbaaSqaaiaadkhaaeqaaOGaam4uamaaDaaaleaacaaIYaGaaGyoaiaaiIdaaeaacaaIWaaaaOGaey4kaSIaeuiLdq0aaSbaaSqaaiaadkhaaeqaaOGaam4yamaaBaaaleaacaWGWbaabeaakiabgwSixlGacYgacaGGUbWaaSaaaeaacaWGubaabaGaaGOmaiaaiMdacaaI4aaaaaqaaiaadkfaaaGaeyypa0dabaGaaGzbVlaaywW7caaMc8Uaeyypa0JaeyOeI0YaaSaaaeaacqqHuoardaWgaaWcbaGaamOCaaqabaGccaWGibWaa0baaSqaaiaaikdacaaI5aGaaGioaaqaaiaaicdaaaGccqGHsislcaaIYaGaaGyoaiaaiIdacqGHflY1cqqHuoardaWgaaWcbaGaamOCaaqabaGccaWGJbWaaSbaaSqaaiaadchaaeqaaaGcbaGaamOuaiaadsfaaaGaey4kaSYaaSaaaeaacqqHuoardaWgaaWcbaGaamOCaaqabaGccaWGJbWaaSbaaSqaaiaadchaaeqaaaGcbaGaamOuaaaaciGGSbGaaiOBaiaadsfacqGHRaWkdaqadaqaamaalaaabaGaeuiLdq0aaSbaaSqaaiaadkhaaeqaaOGaam4yamaaBaaaleaacaWGWbaabeaakmaabmaabaGaaGymaiabgkHiTiGacYgacaGGUbGaaGOmaiaaiMdacaaI4aaacaGLOaGaayzkaaGaey4kaSIaeuiLdq0aaSbaaSqaaiaadkhaaeqaaOGaam4uamaaDaaaleaacaaIYaGaaGyoaiaaiIdaaeaacaaIWaaaaaGcbaGaamOuaaaaaiaawIcacaGLPaaacaGGUaaaaaa@B3E8@
Δ
r
H
298
0
≈(23009+298⋅0,663)R=192,94 кДж/моль.
MathType@MTEF@5@5@+=feaagaart1ev2aaatCvAUfeBSjuyZL2yd9gzLbvyNv2CaerbuLwBLnhiov2DGi1BTfMBaeXatLxBI9gBaerbd9wDYLwzYbItLDharqqtubsr4rNCHbGeaGqiVu0Je9sqqrpepC0xbbL8F4rqqrFfpeea0xe9Lq=Jc9vqaqpepm0xbba9pwe9Q8fs0=yqaqpepae9pg0FirpepeKkFr0xfr=xfr=xb9adbaqaaeGaciGaaiaabeqaamaabaabaaGcbaGaeuiLdq0aaSbaaSqaaiaadkhaaeqaaOGaamisamaaDaaaleaacaaIYaGaaGyoaiaaiIdaaeaacaaIWaaaaOGaeyisISRaaiikaiaaikdacaaIZaGaaGimaiaaicdacaaI5aGaey4kaSIaaGOmaiaaiMdacaaI4aGaeyyXICTaaGimaiaacYcacaaI2aGaaGOnaiaaiodacaGGPaGaamOuaiabg2da9iaaigdacaaI5aGaaGOmaiaacYcacaaI5aGaaGinaiaaykW7caqG6qGaaeifeiaabAdbcaqGVaGaaeipeiaab6dbcaqG7qGaaeiteiaab6caaaa@5AB9@
Энергию связи в современной справочной литературе определяют как
Δ
r
U
298
0
MathType@MTEF@5@5@+=feaagaart1ev2aaatCvAUfeBSjuyZL2yd9gzLbvyNv2CaerbuLwBLnhiov2DGi1BTfMBaeXatLxBI9gBaerbd9wDYLwzYbItLDharqqtubsr4rNCHbGeaGqiVu0Je9sqqrpepC0xbbL8F4rqqrFfpeea0xe9Lq=Jc9vqaqpepm0xbba9pwe9Q8fs0=yqaqpepae9pg0FirpepeKkFr0xfr=xfr=xb9adbaqaaeGaciGaaiaabeqaamaabaabaaGcbaGaeuiLdq0aaSbaaSqaaiaadkhaaeqaaOGaamyvamaaDaaaleaacaaIYaGaaGyoaiaaiIdaaeaacaaIWaaaaaaa@3C82@
(для стандартного состояния вещества):
Δ
r
U
298
0
=
Δ
r
H
298
0
−
Δ
r
(
PV
)
298
=
Δ
r
H
298
0
−298R=190,46 кДж/моль;
MathType@MTEF@5@5@+=feaagaart1ev2aaatCvAUfeBSjuyZL2yd9gzLbvyNv2CaerbuLwBLnhiov2DGi1BTfMBaeXatLxBI9gBaerbd9wDYLwzYbItLDharqqtubsr4rNCHbGeaGqiVu0Je9sqqrpepC0xbbL8F4rqqrFfpeea0xe9Lq=Jc9vqaqpepm0xbba9pwe9Q8fs0=yqaqpepae9pg0FirpepeKkFr0xfr=xfr=xb9adbaqaaeGaciGaaiaabeqaamaabaabaaGcbaGaeuiLdq0aaSbaaSqaaiaadkhaaeqaaOGaamyvamaaDaaaleaacaaIYaGaaGyoaiaaiIdaaeaacaaIWaaaaOGaeyypa0JaeuiLdq0aaSbaaSqaaiaadkhaaeqaaOGaamisamaaDaaaleaacaaIYaGaaGyoaiaaiIdaaeaacaaIWaaaaOGaeyOeI0IaeuiLdq0aaSbaaSqaaiaadkhaaeqaaOWaaeWaaeaacaWGqbGaamOvaaGaayjkaiaawMcaamaaBaaaleaacaaIYaGaaGyoaiaaiIdaaeqaaOGaeyypa0JaeuiLdq0aaSbaaSqaaiaadkhaaeqaaOGaamisamaaDaaaleaacaaIYaGaaGyoaiaaiIdaaeaacaaIWaaaaOGaeyOeI0IaaGOmaiaaiMdacaaI4aGaamOuaiabg2da9iaaigdacaaI5aGaaGimaiaacYcacaaI0aGaaGOnaiaaykW7caaMc8UaaeOoeiaabsbbcaqG2qGaae4laiaabYdbcaqG+qGaae4oeiaabYebcaqG7aaaaa@6819@
либо как
Δ
r
U
0K
0
MathType@MTEF@5@5@+=feaagaart1ev2aaatCvAUfeBSjuyZL2yd9gzLbvyNv2CaerbuLwBLnhiov2DGi1BTfMBaeXatLxBI9gBaerbd9wDYLwzYbItLDharqqtubsr4rNCHbGeaGqiVu0Je9sqqrpepC0xbbL8F4rqqrFfpeea0xe9Lq=Jc9vqaqpepm0xbba9pwe9Q8fs0=yqaqpepae9pg0FirpepeKkFr0xfr=xfr=xb9adbaqaaeGaciGaaiaabeqaamaabaabaaGcbaGaeuiLdq0aaSbaaSqaaiaadkhaaeqaaOGaamyvamaaDaaaleaacaaIWaGaam4saaqaaiaaicdaaaaaaa@3BCB@
= 23009 R = 191,3 кДж/моль
72. (4/1-08).Газообразный углеводород A участвует в двух реакциях, приводящих к получению изомеров В и С:
A ↔ B и A ↔ С.
Значения стандартных энтальпий, энтропий и потенциалов Гиббса образования указанных веществ при 1000 К приведены в таблице
Вещество |
ΔfНo(газ), |
ΔfGo(газ), |
So(газ), |
кДж/моль |
Дж/моль·К |
А (Перилен)
B (Бензопирен – е)
C (Бензопирен – а) |
253,2
253,2
262,4 |
–734,7
–740,5
–737,0 |
987,9
993,7
999,4 |
Определите равновесный состав при 1000 К. Какой из изомеров будет преобладать при последующем повышении температуры?
Решение.
K
P1
=exp(
−
Δ
r1
G
0
RT
)=exp(
−
−740500+734700
8,31⋅1000
)=exp(+0,698)=2.
MathType@MTEF@5@5@+=feaagaart1ev2aaatCvAUfeBSjuyZL2yd9gzLbvyNv2CaerbuLwBLnhiov2DGi1BTfMBaeXatLxBI9gBaerbd9wDYLwzYbItLDharqqtubsr4rNCHbGeaGqiVu0Je9sqqrpepC0xbbL8F4rqqrFfpeea0xe9Lq=Jc9vqaqpepm0xbba9pwe9Q8fs0=yqaqpepae9pg0FirpepeKkFr0xfr=xfr=xb9adbaqaaeGaciGaaiaabeqaamaabaabaaGcbaGaam4samaaBaaaleaacaWGqbGaaGymaaqabaGccqGH9aqpciGGLbGaaiiEaiaacchadaqadaqaaiabgkHiTmaalaaabaGaeuiLdq0aaSbaaSqaaiaadkhacaaIXaaabeaakiaadEeadaahaaWcbeqaaiaaicdaaaaakeaacaWGsbGaamivaaaaaiaawIcacaGLPaaacqGH9aqpciGGLbGaaiiEaiaacchadaqadaqaaiabgkHiTmaalaaabaGaeyOeI0IaaG4naiaaisdacaaIWaGaaGynaiaaicdacaaIWaGaey4kaSIaaG4naiaaiodacaaI0aGaaG4naiaaicdacaaIWaaabaGaaGioaiaacYcacaaIZaGaaGymaiabgwSixlaaigdacaaIWaGaaGimaiaaicdaaaaacaGLOaGaayzkaaGaeyypa0JaciyzaiaacIhacaGGWbGaaiikaiabgUcaRiaaicdacaGGSaGaaGOnaiaaiMdacaaI4aGaaiykaiabg2da9iaaikdacaGGUaaaaa@6B14@
K
P2
=exp(
−
Δ
r2
G
0
RT
)=exp(
−
−737000+734700
8,31⋅1000
)=exp(+0,277)=1,32.
MathType@MTEF@5@5@+=feaagaart1ev2aaatCvAUfeBSjuyZL2yd9gzLbvyNv2CaerbuLwBLnhiov2DGi1BTfMBaeXatLxBI9gBaerbd9wDYLwzYbItLDharqqtubsr4rNCHbGeaGqiVu0Je9sqqrpepC0xbbL8F4rqqrFfpeea0xe9Lq=Jc9vqaqpepm0xbba9pwe9Q8fs0=yqaqpepae9pg0FirpepeKkFr0xfr=xfr=xb9adbaqaaeGaciGaaiaabeqaamaabaabaaGcbaGaam4samaaBaaaleaacaWGqbGaaGOmaaqabaGccqGH9aqpciGGLbGaaiiEaiaacchadaqadaqaaiabgkHiTmaalaaabaGaeuiLdq0aaSbaaSqaaiaadkhacaaIYaaabeaakiaadEeadaahaaWcbeqaaiaaicdaaaaakeaacaWGsbGaamivaaaaaiaawIcacaGLPaaacqGH9aqpciGGLbGaaiiEaiaacchadaqadaqaaiabgkHiTmaalaaabaGaeyOeI0IaaG4naiaaiodacaaI3aGaaGimaiaaicdacaaIWaGaey4kaSIaaG4naiaaiodacaaI0aGaaG4naiaaicdacaaIWaaabaGaaGioaiaacYcacaaIZaGaaGymaiabgwSixlaaigdacaaIWaGaaGimaiaaicdaaaaacaGLOaGaayzkaaGaeyypa0JaciyzaiaacIhacaGGWbGaaiikaiabgUcaRiaaicdacaGGSaGaaGOmaiaaiEdacaaI3aGaaiykaiabg2da9iaaigdacaGGSaGaaG4maiaaikdacaGGUaaaaa@6D38@
Состав смеси:
x
A
=
P
A
P
A
+
P
B
+
P
C
=
1
1+
K
P1
+
K
P2
=0,23
MathType@MTEF@5@5@+=feaagaart1ev2aaatCvAUfeBSjuyZL2yd9gzLbvyNv2CaerbuLwBLnhiov2DGi1BTfMBaeXatLxBI9gBaerbd9wDYLwzYbItLDharqqtubsr4rNCHbGeaGqiVu0Je9sqqrpepC0xbbL8F4rqqrFfpeea0xe9Lq=Jc9vqaqpepm0xbba9pwe9Q8fs0=yqaqpepae9pg0FirpepeKkFr0xfr=xfr=xb9adbaqaaeGaciGaaiaabeqaamaabaabaaGcbaGaamiEamaaBaaaleaacaWGbbaabeaakiabg2da9maalaaabaGaamiuamaaBaaaleaacaWGbbaabeaaaOqaaiaadcfadaWgaaWcbaGaamyqaaqabaGccqGHRaWkcaWGqbWaaSbaaSqaaiaadkeaaeqaaOGaey4kaSIaamiuamaaBaaaleaacaWGdbaabeaaaaGccqGH9aqpdaWcaaqaaiaaigdaaeaacaaIXaGaey4kaSIaam4samaaBaaaleaacaWGqbGaaGymaaqabaGccqGHRaWkcaWGlbWaaSbaaSqaaiaadcfacaaIYaaabeaaaaGccqGH9aqpcaaIWaGaaiilaiaaikdacaaIZaaaaa@4F6D@
x
B
=
P
B
P
A
+
P
B
+
P
C
=
K
P1
1+
K
P1
+
K
P2
=0,46
MathType@MTEF@5@5@+=feaagaart1ev2aaatCvAUfeBSjuyZL2yd9gzLbvyNv2CaerbuLwBLnhiov2DGi1BTfMBaeXatLxBI9gBaerbd9wDYLwzYbItLDharqqtubsr4rNCHbGeaGqiVu0Je9sqqrpepC0xbbL8F4rqqrFfpeea0xe9Lq=Jc9vqaqpepm0xbba9pwe9Q8fs0=yqaqpepae9pg0FirpepeKkFr0xfr=xfr=xb9adbaqaaeGaciGaaiaabeqaamaabaabaaGcbaGaamiEamaaBaaaleaacaWGcbaabeaakiabg2da9maalaaabaGaamiuamaaBaaaleaacaWGcbaabeaaaOqaaiaadcfadaWgaaWcbaGaamyqaaqabaGccqGHRaWkcaWGqbWaaSbaaSqaaiaadkeaaeqaaOGaey4kaSIaamiuamaaBaaaleaacaWGdbaabeaaaaGccqGH9aqpdaWcaaqaaiaadUeadaWgaaWcbaGaamiuaiaaigdaaeqaaaGcbaGaaGymaiabgUcaRiaadUeadaWgaaWcbaGaamiuaiaaigdaaeqaaOGaey4kaSIaam4samaaBaaaleaacaWGqbGaaGOmaaqabaaaaOGaeyypa0JaaGimaiaacYcacaaI0aGaaGOnaaaa@514F@
x
C
=
P
C
P
A
+
P
B
+
P
C
=
K
P2
1+
K
P1
+
K
P2
=0,3
MathType@MTEF@5@5@+=feaagaart1ev2aaatCvAUfeBSjuyZL2yd9gzLbvyNv2CaerbuLwBLnhiov2DGi1BTfMBaeXatLxBI9gBaerbd9wDYLwzYbItLDharqqtubsr4rNCHbGeaGqiVu0Je9sqqrpepC0xbbL8F4rqqrFfpeea0xe9Lq=Jc9vqaqpepm0xbba9pwe9Q8fs0=yqaqpepae9pg0FirpepeKkFr0xfr=xfr=xb9adbaqaaeGaciGaaiaabeqaamaabaabaaGcbaGaamiEamaaBaaaleaacaWGdbaabeaakiabg2da9maalaaabaGaamiuamaaBaaaleaacaWGdbaabeaaaOqaaiaadcfadaWgaaWcbaGaamyqaaqabaGccqGHRaWkcaWGqbWaaSbaaSqaaiaadkeaaeqaaOGaey4kaSIaamiuamaaBaaaleaacaWGdbaabeaaaaGccqGH9aqpdaWcaaqaaiaadUeadaWgaaWcbaGaamiuaiaaikdaaeqaaaGcbaGaaGymaiabgUcaRiaadUeadaWgaaWcbaGaamiuaiaaigdaaeqaaOGaey4kaSIaam4samaaBaaaleaacaWGqbGaaGOmaaqabaaaaOGaeyypa0JaaGimaiaacYcacaaIZaaaaa@5091@
При увеличении температуры:
∂
∂T
(
x
B
x
A
)=
∂
∂T
(
P
B
P
A
)=
∂
K
P1
∂T
=
K
P1
Δ
r1
H
0
R
T
2
=0.
MathType@MTEF@5@5@+=feaagaart1ev2aaatCvAUfeBSjuyZL2yd9gzLbvyNv2CaerbuLwBLnhiov2DGi1BTfMBaeXatLxBI9gBaerbd9wDYLwzYbItLDharqqtubsr4rNCHbGeaGqiVu0Je9sqqrpepC0xbbL8F4rqqrFfpeea0xe9Lq=Jc9vqaqpepm0xbba9pwe9Q8fs0=yqaqpepae9pg0FirpepeKkFr0xfr=xfr=xb9adbaqaaeGaciGaaiaabeqaamaabaabaaGcbaWaaSaaaeaacqGHciITaeaacqGHciITcaWGubaaamaabmaabaWaaSaaaeaacaWG4bWaaSbaaSqaaiaadkeaaeqaaaGcbaGaamiEamaaBaaaleaacaWGbbaabeaaaaaakiaawIcacaGLPaaacqGH9aqpdaWcaaqaaiabgkGi2cqaaiabgkGi2kaadsfaaaWaaeWaaeaadaWcaaqaaiaadcfadaWgaaWcbaGaamOqaaqabaaakeaacaWGqbWaaSbaaSqaaiaadgeaaeqaaaaaaOGaayjkaiaawMcaaiabg2da9maalaaabaGaeyOaIyRaam4samaaBaaaleaacaWGqbGaaGymaaqabaaakeaacqGHciITcaWGubaaaiabg2da9iaadUeadaWgaaWcbaGaamiuaiaaigdaaeqaaOWaaSaaaeaacqqHuoardaWgaaWcbaGaamOCaiaaigdaaeqaaOGaamisamaaCaaaleqabaGaaGimaaaaaOqaaiaadkfacaWGubWaaWbaaSqabeaacaaIYaaaaaaakiabg2da9iaaicdacaGGUaaaaa@5E43@
∂
∂T
(
x
C
x
A
)=
∂
∂T
(
P
C
P
A
)=
∂
K
P2
∂T
=
K
P2
Δ
r2
H
0
R
T
2
=
K
P2
⋅1,1⋅
10
−3
> 0.
MathType@MTEF@5@5@+=feaagaart1ev2aaatCvAUfeBSjuyZL2yd9gzLbvyNv2CaerbuLwBLnhiov2DGi1BTfMBaeXatLxBI9gBaerbd9wDYLwzYbItLDharqqtubsr4rNCHbGeaGqiVu0Je9sqqrpepC0xbbL8F4rqqrFfpeea0xe9Lq=Jc9vqaqpepm0xbba9pwe9Q8fs0=yqaqpepae9pg0FirpepeKkFr0xfr=xfr=xb9adbaqaaeGaciGaaiaabeqaamaabaabaaGcbaWaaSaaaeaacqGHciITaeaacqGHciITcaWGubaaamaabmaabaWaaSaaaeaacaWG4bWaaSbaaSqaaiaadoeaaeqaaaGcbaGaamiEamaaBaaaleaacaWGbbaabeaaaaaakiaawIcacaGLPaaacqGH9aqpdaWcaaqaaiabgkGi2cqaaiabgkGi2kaadsfaaaWaaeWaaeaadaWcaaqaaiaadcfadaWgaaWcbaGaam4qaaqabaaakeaacaWGqbWaaSbaaSqaaiaadgeaaeqaaaaaaOGaayjkaiaawMcaaiabg2da9maalaaabaGaeyOaIyRaam4samaaBaaaleaacaWGqbGaaGOmaaqabaaakeaacqGHciITcaWGubaaaiabg2da9iaadUeadaWgaaWcbaGaamiuaiaaikdaaeqaaOWaaSaaaeaacqqHuoardaWgaaWcbaGaamOCaiaaikdaaeqaaOGaamisamaaCaaaleqabaGaaGimaaaaaOqaaiaadkfacaWGubWaaWbaaSqabeaacaaIYaaaaaaakiabg2da9iaadUeadaWgaaWcbaGaamiuaiaaikdaaeqaaOGaeyyXICTaaGymaiaacYcacaaIXaGaeyyXICTaaGymaiaaicdadaahaaWcbeqaaiabgkHiTiaaiodaaaGccaaMc8UaeyOpa4JaaGjbVlaaicdacaGGUaaaaa@6F0F@
В равновесной смеси при повышении температуры будет повышаться мольная доля вещества С, соотношение B:A будет постоянным. Однако рост KP2 очень “медленный” – порядка 0,1 % на 1 K, поэтому преобладать будет изомер B.
96. (4/2-00).Для систем
CuSO4×
5H2O = CuSO4×
3H2O + 2H2O (1)
CuSO4×
3H2O = CuSO4×
H2O + 2H2O (2)
CuSO4×
H2O = CuSO4 + H2O (3)
давление насыщенного пара при 50 °С равно соответственно 47, 30 и 4,4 торр.
В герметичный сосуд небольшого объема поместили 1 моль безводного сульфата меди, откачали и затем стали вводить пары воды при данной температуре. Нарисовать зависимость давления паров воды в системе от количества введенных молей воды. Пояснить приведенный рисунок.
Решение. При давлении паров воды менее 4,4 торр, насыщение парами воды не достигается ни д
ля одной реакции. Давление паров воды растет пропорционально количеству введенной воды.
После достижения давления 4,4 торр (nH2O = δ) равновесно сосуществуют моногидрат
сульфата меди и безводный сульфат, соотношение этих фаз определяется количеством введенной воды,
но, пока сосуществуют обе фазы, давление паров воды составляет 4,4 торр. Максимальное количество
воды, при вводе которого РH2O = 4,4 торр, составяет δ + 1 моль.
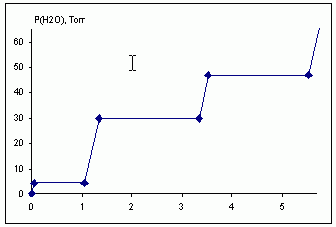
Дальнейшее добавление в систему воды приведет к дальнейшему росту давления паров воды, пока не будет достигнуто давление 30 торр (количество введенной воды составит 6,8δ + 1 моль). При дальнейшем добавлении воды происходит превращение моногидрата в тригидрат. Сосуществование этих фаз в равновесии требует давления паров воды 30 торр. Полное превращение моногидрата в тригидрат будет достигнуто при nH2O = 6,8.δ + 3 моль.
При достижении давления 47 торр в равновесии находится реакция (1). Равновесное давление 47 торр поддерживается в диапазоне введенной воды от 10,7.δ + 3 моль до 10,7.δ + 5 моль. Дальше давление паров воды опять будет линейно повышаться с увеличением количества введенной воды.
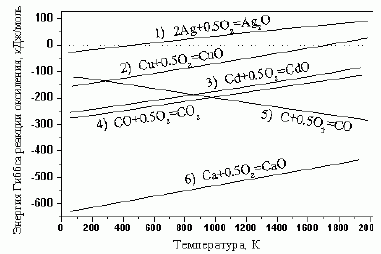
107. (2/1-07).Зависимости
Δ
r
G
0
(T)
MathType@MTEF@5@5@+=feaagaart1ev2aaatCvAUfeBSjuyZL2yd9gzLbvyNv2CaerbuLwBLnhiov2DGi1BTfMBaeXatLxBI9gBaerbd9wDYLwzYbItLDharqqtubsr4rNCHbGeaGqiVu0Je9sqqrpepC0xbbL8F4rqqrFfpeea0xe9Lq=Jc9vqaqpepm0xbba9pwe9Q8fs0=yqaqpepae9pg0FirpepeKkFr0xfr=xfr=xb9adbaqaaeGaciGaaiaabeqaamaabaabaaGcbaGaeuiLdq0aaSbaaSqaaiaadkhaaeqaaOGaam4ramaaCaaaleqabaGaaGimaaaakiaacIcacaWGubGaaiykaaaa@3C6F@
реакций окисления ряда металлов, графита и СО (диаграммы Эллингхэма) приведены на рисунке. Определите: 1) при какой T и какие металлы могут самопроизвольно восста-навливаться из соответ-ствующих оксидов;
2) при какой T и какие металлы можно восста-новить монооксидом углерода;
3) при какой T и какие металлы можно восстано-вить графитом?
Парциальные давления всех газообразных веществ считать равными 1 атм.
Решение. Условие самопроизвольного процесса
Δ
r
G
<0
MathType@MTEF@5@5@+=feaagaart1ev2aaatCvAUfeBSjuyZL2yd9gzLbvyNv2CaerbuLwBLnhiov2DGi1BTfMBaeXatLxBI9gBaerbd9wDYLwzYbItLDharqqtubsr4rNCHbGeaGqiVu0Je9sqqrpepC0xbbL8F4rqqrFfpeea0xe9Lq=Jc9vqaqpepm0xbba9pwe9Q8fs0=yqaqpepae9pg0FirpepeKkFr0xfr=xfr=xb9adbaqaaeGaciGaaiaabeqaamaabaabaaGcbaGaeuiLdq0aaSbaaSqaaiaadkhaaeqaaOGaam4ramaaCaaaleqabaaaaOGaeyipaWJaaGimaaaa@3B41@
, при парциальных давлениях 1 атм это условие переходит в
Δ
r
G
0
<0
MathType@MTEF@5@5@+=feaagaart1ev2aaatCvAUfeBSjuyZL2yd9gzLbvyNv2CaerbuLwBLnhiov2DGi1BTfMBaeXatLxBI9gBaerbd9wDYLwzYbItLDharqqtubsr4rNCHbGeaGqiVu0Je9sqqrpepC0xbbL8F4rqqrFfpeea0xe9Lq=Jc9vqaqpepm0xbba9pwe9Q8fs0=yqaqpepae9pg0FirpepeKkFr0xfr=xfr=xb9adbaqaaeGaciGaaiaabeqaamaabaabaaGcbaGaeuiLdq0aaSbaaSqaaiaadkhaaeqaaOGaam4ramaaCaaaleqabaGaaGimaaaakiabgYda8iaaicdaaaa@3BFB@
.
Самопроизвольно восстанавливаются:
Ag при Т > 490 К по реакции Ag2O = 2Ag + 0,5O2,
−
Δ
r1
G
0
(T>490)<0
MathType@MTEF@5@5@+=feaagaart1ev2aaatCvAUfeBSjuyZL2yd9gzLbvyNv2CaerbuLwBLnhiov2DGi1BTfMBaeXatLxBI9gBaerbd9wDYLwzYbItLDharqqtubsr4rNCHbGeaGqiVu0Je9sqqrpepC0xbbL8F4rqqrFfpeea0xe9Lq=Jc9vqaqpepm0xbba9pwe9Q8fs0=yqaqpepae9pg0FirpepeKkFr0xfr=xfr=xb9adbaqaaeGaciGaaiaabeqaamaabaabaaGcbaGaeyOeI0IaeuiLdq0aaSbaaSqaaiaadkhacaaIXaaabeaakiaadEeadaqhaaWcbaaabaGaaGimaaaakiaacIcacaWGubGaeyOpa4JaaGinaiaaiMdacaaIWaGaaiykaiabgYda8iaaicdaaaa@4318@
и Cu при Т > 1720 К по реакции CuO = Cu + 0,5O2,
−
Δ
r2
G
0
(T>1720)<0
MathType@MTEF@5@5@+=feaagaart1ev2aaatCvAUfeBSjuyZL2yd9gzLbvyNv2CaerbuLwBLnhiov2DGi1BTfMBaeXatLxBI9gBaerbd9wDYLwzYbItLDharqqtubsr4rNCHbGeaGqiVu0Je9sqqrpepC0xbbL8F4rqqrFfpeea0xe9Lq=Jc9vqaqpepm0xbba9pwe9Q8fs0=yqaqpepae9pg0FirpepeKkFr0xfr=xfr=xb9adbaqaaeGaciGaaiaabeqaamaabaabaaGcbaGaeyOeI0IaeuiLdq0aaSbaaSqaaiaadkhacaaIYaaabeaakiaadEeadaqhaaWcbaaabaGaaGimaaaakiaacIcacaWGubGaeyOpa4JaaGymaiaaiEdacaaIYaGaaGimaiaacMcacqGH8aapcaaIWaaaaa@43D0@
. Cd и Ca не восстанавливаются.
Монооксидом углерода восстанавливаются Ag, Cu и Сd при любой температуре: Ag2O +CO =
2Ag + CO2,
Δ
r
G
0
=
Δ
r4
G
0
−
Δ
r1
G
0
<0
MathType@MTEF@5@5@+=feaagaart1ev2aaatCvAUfeBSjuyZL2yd9gzLbvyNv2CaerbuLwBLnhiov2DGi1BTfMBaeXatLxBI9gBaerbd9wDYLwzYbItLDharqqtubsr4rNCHbGeaGqiVu0Je9sqqrpepC0xbbL8F4rqqrFfpeea0xe9Lq=Jc9vqaqpepm0xbba9pwe9Q8fs0=yqaqpepae9pg0FirpepeKkFr0xfr=xfr=xb9adbaqaaeGaciGaaiaabeqaamaabaabaaGcbaGaeuiLdq0aaSbaaSqaaiaadkhaaeqaaOGaam4ramaaCaaaleqabaGaaGimaaaakiabg2da9iabfs5aenaaBaaaleaacaWGYbGaaGinaaqabaGccaWGhbWaa0baaSqaaaqaaiaaicdaaaGccqGHsislcqqHuoardaWgaaWcbaGaamOCaiaaigdaaeqaaOGaam4ramaaDaaaleaaaeaacaaIWaaaaOGaeyipaWJaaGimaaaa@4807@
,
CuO + CO = Cu + CO2,
Δ
r
G
0
=
Δ
r4
G
0
−
Δ
r2
G
0
<0
MathType@MTEF@5@5@+=feaagaart1ev2aaatCvAUfeBSjuyZL2yd9gzLbvyNv2CaerbuLwBLnhiov2DGi1BTfMBaeXatLxBI9gBaerbd9wDYLwzYbItLDharqqtubsr4rNCHbGeaGqiVu0Je9sqqrpepC0xbbL8F4rqqrFfpeea0xe9Lq=Jc9vqaqpepm0xbba9pwe9Q8fs0=yqaqpepae9pg0FirpepeKkFr0xfr=xfr=xb9adbaqaaeGaciGaaiaabeqaamaabaabaaGcbaGaeuiLdq0aaSbaaSqaaiaadkhaaeqaaOGaam4ramaaCaaaleqabaGaaGimaaaakiabg2da9iabfs5aenaaBaaaleaacaWGYbGaaGinaaqabaGccaWGhbWaa0baaSqaaaqaaiaaicdaaaGccqGHsislcqqHuoardaWgaaWcbaGaamOCaiaaikdaaeqaaOGaam4ramaaDaaaleaaaeaacaaIWaaaaOGaeyipaWJaaGimaaaa@4808@
,
Δ
r
G
0
=
Δ
r4
G
0
−
Δ
r3
G
0
<0
MathType@MTEF@5@5@+=feaagaart1ev2aaatCvAUfeBSjuyZL2yd9gzLbvyNv2CaerbuLwBLnhiov2DGi1BTfMBaeXatLxBI9gBaerbd9wDYLwzYbItLDharqqtubsr4rNCHbGeaGqiVu0Je9sqqrpepC0xbbL8F4rqqrFfpeea0xe9Lq=Jc9vqaqpepm0xbba9pwe9Q8fs0=yqaqpepae9pg0FirpepeKkFr0xfr=xfr=xb9adbaqaaeGaciGaaiaabeqaamaabaabaaGcbaGaeuiLdq0aaSbaaSqaaiaadkhaaeqaaOGaam4ramaaCaaaleqabaGaaGimaaaakiabg2da9iabfs5aenaaBaaaleaacaWGYbGaaGinaaqabaGccaWGhbWaa0baaSqaaaqaaiaaicdaaaGccqGHsislcqqHuoardaWgaaWcbaGaamOCaiaaiodaaeqaaOGaam4ramaaDaaaleaaaeaacaaIWaaaaOGaeyipaWJaaGimaaaa@4809@
.
Не восстанавливается кальций.
Графитом восстанавливаются:
Ag, при любой T: Ag2O + C = 2Ag + CO,
Δ
r
G
0
=
Δ
r5
G
0
−
Δ
r1
G
0
<0
MathType@MTEF@5@5@+=feaagaart1ev2aaatCvAUfeBSjuyZL2yd9gzLbvyNv2CaerbuLwBLnhiov2DGi1BTfMBaeXatLxBI9gBaerbd9wDYLwzYbItLDharqqtubsr4rNCHbGeaGqiVu0Je9sqqrpepC0xbbL8F4rqqrFfpeea0xe9Lq=Jc9vqaqpepm0xbba9pwe9Q8fs0=yqaqpepae9pg0FirpepeKkFr0xfr=xfr=xb9adbaqaaeGaciGaaiaabeqaamaabaabaaGcbaGaeuiLdq0aaSbaaSqaaiaadkhaaeqaaOGaam4ramaaCaaaleqabaGaaGimaaaakiabg2da9iabfs5aenaaBaaaleaacaWGYbGaaGynaaqabaGccaWGhbWaa0baaSqaaaqaaiaaicdaaaGccqGHsislcqqHuoardaWgaaWcbaGaamOCaiaaigdaaeqaaOGaam4ramaaDaaaleaaaeaacaaIWaaaaOGaeyipaWJaaGimaaaa@4808@
,
Cu при Т > 280 К: CuO + C = Cu + CO,
Δ
r
G
0
=
Δ
r5
G
0
−
Δ
r2
G
0
<0
MathType@MTEF@5@5@+=feaagaart1ev2aaatCvAUfeBSjuyZL2yd9gzLbvyNv2CaerbuLwBLnhiov2DGi1BTfMBaeXatLxBI9gBaerbd9wDYLwzYbItLDharqqtubsr4rNCHbGeaGqiVu0Je9sqqrpepC0xbbL8F4rqqrFfpeea0xe9Lq=Jc9vqaqpepm0xbba9pwe9Q8fs0=yqaqpepae9pg0FirpepeKkFr0xfr=xfr=xb9adbaqaaeGaciGaaiaabeqaamaabaabaaGcbaGaeuiLdq0aaSbaaSqaaiaadkhaaeqaaOGaam4ramaaCaaaleqabaGaaGimaaaakiabg2da9iabfs5aenaaBaaaleaacaWGYbGaaGynaaqabaGccaWGhbWaa0baaSqaaaqaaiaaicdaaaGccqGHsislcqqHuoardaWgaaWcbaGaamOCaiaaikdaaeqaaOGaam4ramaaDaaaleaaaeaacaaIWaaaaOGaeyipaWJaaGimaaaa@4809@
,
Cd при Т > 850 К: CdO + C = Cd + CO,
Δ
r
G
0
=
Δ
r5
G
0
−
Δ
r3
G
0
<0
MathType@MTEF@5@5@+=feaagaart1ev2aaatCvAUfeBSjuyZL2yd9gzLbvyNv2CaerbuLwBLnhiov2DGi1BTfMBaeXatLxBI9gBaerbd9wDYLwzYbItLDharqqtubsr4rNCHbGeaGqiVu0Je9sqqrpepC0xbbL8F4rqqrFfpeea0xe9Lq=Jc9vqaqpepm0xbba9pwe9Q8fs0=yqaqpepae9pg0FirpepeKkFr0xfr=xfr=xb9adbaqaaeGaciGaaiaabeqaamaabaabaaGcbaGaeuiLdq0aaSbaaSqaaiaadkhaaeqaaOGaam4ramaaCaaaleqabaGaaGimaaaakiabg2da9iabfs5aenaaBaaaleaacaWGYbGaaGynaaqabaGccaWGhbWaa0baaSqaaaqaaiaaicdaaaGccqGHsislcqqHuoardaWgaaWcbaGaamOCaiaaiodaaeqaaOGaam4ramaaDaaaleaaaeaacaaIWaaaaOGaeyipaWJaaGimaaaa@480A@
.
Не восстанавливается кальций.


